MOLNUPIRAVIR
Molnupiravir is an antiviral drug which inhibits viral reproduction by promoting widespread mutations in the replication of viral RNA by RNA-directed RNA polymerase. It is used to treat COVID-19 in those infected by SARS-CoV-2. It is indicated for adult Covid-19 patients having 93% blood oxygen levels.
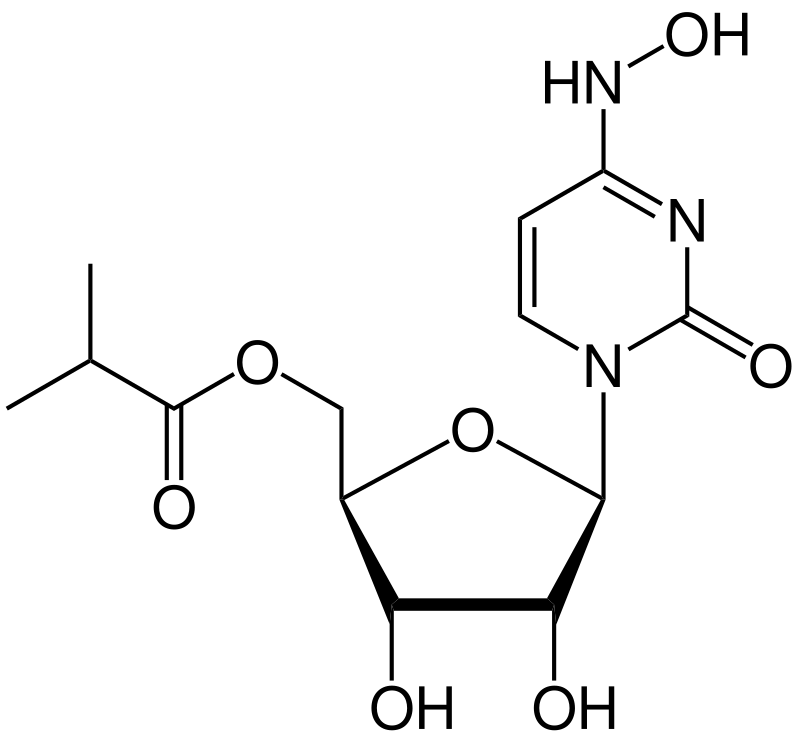
It is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine and exerts its antiviral action through introduction of copying errors during viral RNA replication.
Originally the drug was developed to treat influenza at Emory University by the university’s drug innovation company, Drug Innovation Ventures at Emory (DRIVE), but was reportedly abandoned for mutagenicity concerns. Later it was acquired by Miam based company Ridge back Biotherapeutics, which later partnered with Merck & Co. to develop the drug further.
Based on positive results in placebo-controlled double-blind randomized clinical trials, Molnupiravir was approved for medical use in the United Kingdom in November 2021. In December 2021, the U.S. Food and Drug Administration (FDA) granted an emergency use authorization (EUA) to molnupiravir for use in certain populations where other treatments are not feasible.
Molnupiravir is indicated for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19.
The drug is administered as four 200 mg capsules taken orally every 12 hours for five days, for a total of 40 capsules. It is not authorized for use for longer than five consecutive days.
13 Indian drug manufactures will be producing the drug domestically. Dr. Reddy Laboratories, Cipla, Natco Pharma, Optimus Pharma Pvt Ltd, Stride, and, Hetero are among the large drug manufacturers.
Its use in pregnancy is not recommended. There are no human data on use during pregnancy to assess the risk of adverse maternal or fetal outcomes. Based on animal data, the drug may cause fetal harm. Breastfeeding is not recommended during treatment due to potential adverse reactions in the infant.
There are no data on the presence of the drug or its metabolites in human milk. It is not known whether it has an effect on the infant or on milk production. Use in patients under 18 years of age can affect bone and cartilage growth. In rats, bone and cartilage toxicity was observed after repeated dosing.